>> In the charged particle scattering experiment, the particle that is repelled back from the gold nucleus will have to be at rest before it changes direction. Rutherford proposes that he could use this fact (energy conservation in this situation) to estimate the size of the nucleus. This is because when the particle is at rest, its KE will be balanced by the electric potential energy due to the repulsive electrostatic force.


13.2.2. Describe how the masses of nuclei may be determined using a Bainbridge mass spectrometer.
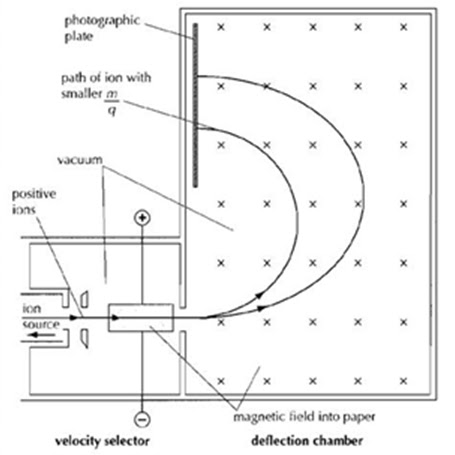
>> The principle of Bainbridge mass spectrometer is to use the magnetic field to deflect moving ions of a substance. If a moving ion enters a constant magnetic field, B, it will follow a circular path. Because the radius of the circular path depends on the mass of the ion, a larger mass ion will travel in a larger circular path. Thus the principle can be used to determine the masses of nuclei:

please show the formula to work out the closest approach for 13.2.1
ReplyDelete